Outcomes for children and adolescents with newly diagnosed acute myeloid leukemia (AML) have only shown modest improvement over the past three decades. Additionally, outcomes for relapsed disease continue to be poor. As a result, there is a pressing need to optimize consolidation therapy to ensure deep and persistent remissions and prevent future relapse. In particular, the utility of bone and marrow transplant (BMT) for consolidation in pediatric AML has been debated for decades. A prior Children's Oncology Group (COG) study risk-stratified patients on the basis of cytogenetic alterations and demonstrated that consolidative BMT performed in first complete remission (CR1) improved long-term outcomes specifically in patients diagnosed with intermediate-risk disease, but found no survival benefit in patients with either low- or high-risk disease. Since then, whole genome and transcriptome sequencing have uncovered a large number of somatic mutations and cryptic fusions that carry prognostic significance in pediatric AML. Combining these molecular features with traditional cytogenetic risk stratification results in the re-classification of a significant number of patients. While this cytomolecular risk stratification is now being used in contemporary clinical trials (e.g., AAML1831/NCT04293562), the merits of consolidative BMT have not been rigorously re-assessed using these newly defined risk strata.
To address this gap, we analyzed clinical and biological data from patients enrolled on COG phase 3 clinical trials AAML0531/NCT00372593 and AAML1031/NCT01371981. We filtered the resulting 2,024 patients to include those who had sufficient diagnostic data to ascertain their cytomolecular risk stratification. Additional inclusion criteria were achieving CR1; completing induction I, induction II, and intensification I; and undergoing BMT after intensification I, which was the recommended time point for BMT on both protocols. Our final analysis included 1,004 patients who met all inclusion criteria. BMT versus chemotherapy alone in CR1 was recommended based on indications provided by AAML0531 or AAML1031, which differ from one another (e.g., AAML1031 incorporated measurable residual disease). Clinical outcomes were assessed using Kaplan-Meier analysis and differences between groups were determined using log-rank testing.
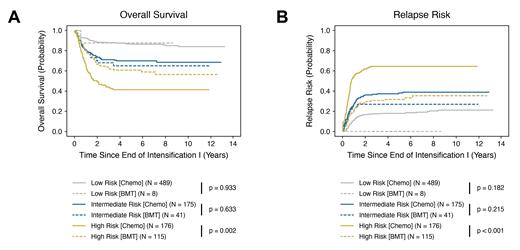
Clinical outcomes were superior for patients in the cytomolecular high-risk cohort who underwent BMT in CR1 relative to those who received chemotherapy alone. Specifically, high-risk patients who underwent BMT had a significantly improved five-year OS of 60.8 ± 9.3% (versus 41.3 ± 7.7%; p = 0.002) ( Fig. 1A) and DFS of 55.9 ± 9.4% (versus 30.6 ± 7.2%; p < 0.001) (data not shown). We also observed a decreased incidence of relapse in high-risk patients who received BMT, with a RR of 31.8 ± 8.8% relative to 64.5 ± 7.5% for patients in the chemotherapy only group (p < 0.001) ( Fig. 1B). Conversely, there was no difference in any of these outcome measures for patients in the low- and intermediate-risk groups who underwent BMT in CR1 versus receiving chemotherapy alone. Subsequent and ongoing analysis preliminarily indicates that while a subset of high-risk cytomolecular subtypes benefit from BMT in CR1, others do not.
Increased availability of high-throughput sequencing has facilitated the identification of numerous molecular features that have prognostic importance in pediatric AML, leading to more accurate risk stratification and opportunities to better allocate patients to appropriate risk-adapted therapy. In this study, we demonstrate that incorporation of these molecular features into the traditional risk stratification schema significantly impacts the clinical indications for BMT as consolidation therapy in pediatric AML. Whereas BMT was previously found to benefit only those patients with intermediate-risk disease, our data suggest that patients with contemporary high-risk cytomolecular alterations benefit significantly from consolidative BMT. Notably, our current analysis does not yet address the potential bias that patients who reach BMT systematically differ from those patients who did not reach BMT (e.g., toxicity that precludes BMT), which we will address with multivariable analysis using BMT as a time varying exposure. These findings support the need for further prospective studies evaluating the role of BMT in high-risk pediatric AML.
Disclosures
No relevant conflicts of interest to declare.